Theragnostics in the Wild: How Real-World Oncology Trials Are Rewriting the Radiopharmaceutical Playbook

Intro
Radiopharmaceuticals are no longer confined to theoretical frameworks or early-phase exploration. SNMMI 2025 highlighted how oncology theragnostic trials are shifting into real-world application—where clinical design, imaging integration, and operational workflows converge to support scalable, evidence-based programs.
These developments signal meaningful adjustments in how trials are being constructed, delivered, and evaluated.
Flexible Dosing Models Require New Infrastructure
The FLEX-MRT Phase 2 study presented at SNMMI 2025 explored an adaptive approach to administering Pluvicto® (177Lu-PSMA-617), allowing for up to 12 treatment cycles based on PSA and imaging criteria [1][6]. This marks a departure from the traditional 6 treatment-cycle standard and introduces additional flexibility and decision points throughout the treatment timeline.
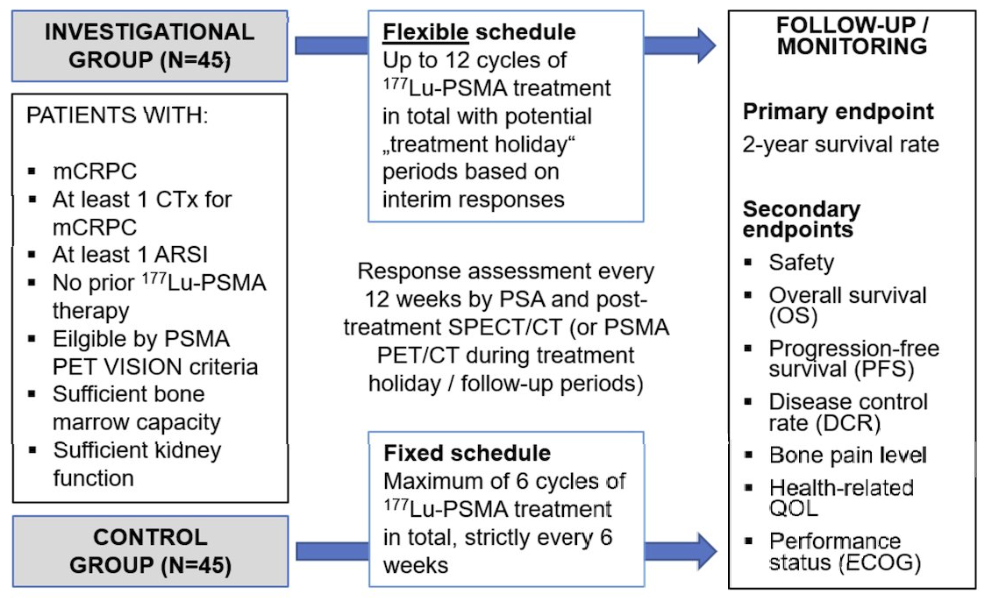
Similarly, early findings from the VIOLET Phase 1/2 study offered insight into [161Tb] -PSMA-I&T, a dual-particle-emitting beta therapy that may offer more concentrated therapeutic effects while maintaining safety [2][7][8]. While early, these data reflect a growing interest in refining isotope selection and dosing strategy based on patient and tumor characteristics.
Theragnostic Insight: When considering personalized treatment models, teams should be prepared to coordinate more complex imaging schedules and real-time data reviews. Operational clarity is essential, especially at sites unfamiliar with flexible radionuclide workflows.
Imaging-Guided Stratification Is Addressing an Unmet Clinical Need
One SNMMI 2025 presentation proposed an automated imaging workflow that uses PSMA PET/CT metrics, such as lesion volume, distribution, and uptake, to help predict progression-free and overall survival in advanced prostate cancer [3]. Rather than functioning solely as a risk score, the model offers a more systematic way to stratify patients and evaluate therapeutic benefit, helping address a long-standing need for clinically actionable imaging tools.
Theragnostic Insight: Imaging-informed prediction models can support more consistent decision-making across sites and trials. Teams should consider how automated metrics might complement existing endpoints and inform discussions with regulators and investigators alike.
Response Assessment Is Evolving Beyond RECIST v1.1?
Conventional response criteria such as RECISTv1.1 do not always capture the therapeutic dynamics of radiopharmaceuticals. At SNMMI 2025, several discussions highlighted the growing use of same-day post-treatment SPECT/CT imaging following Pluvicto® administration to assess uptake and retention. These insights echo findings from The Journal of Nuclear Medicine, where nearly half of patients had their management plans adjusted based on post-SPECT/CT scans—most often at cycles 2 and 4 [4][9]. As new imaging-driven endpoints emerge, they are being considered not just as adjuncts to traditional assessments, but as decision tools in their own right.
Theragnostic Insight: Radiopharmaceutical response assessment may require methods beyond traditional RECIST criteria. Teams should evaluate emerging imaging strategies—such as posttreatment SPECT/CT—not only for regulatory alignment but also for their potential to improve clinical decision-making and patient outcomes.
Real-World Data and Sequential Strategies Are Informing Development
Clinical experience gathered from expanded-access settings is beginning to influence trial strategy. For example, WB SPECT/CT monitoring is being used to guide dose continuation and assess early signs of progression [4]. This real-world feedback loop is enabling more refined patient management in ongoing trials.
Another emerging approach is the sequencing of beta and alpha therapies, such as alternating Pluvicto® with Xofigo® [5]. These combinations are being explored not just for efficacy, but also for potential resistance management and durability of response.
Theragnostic Insight: As sequencing strategies take shape, sponsors will need to anticipate data integration challenges across different agents, timelines, and biomarkers. Pre-specifying these workflows can prevent ambiguity during execution and review.
What This Means for Development Teams
The field is moving away from rigid trial frameworks toward designs that incorporate flexibility, imaging-based personalization, and iterative decision-making. These strategies are now active components of radiopharmaceutical programs entering or advancing through clinical trials.
For sponsors and developers, this shift brings new expectations around coordination, data standardization, and cross-functional alignment. Programs must be engineered not only for regulatory approval, but also for delivery at scale across diverse clinical settings.
At Theragnostic Insights, we support radiopharmaceutical teams through each phase of this evolution—from initial design to operational readiness and beyond. Our guidance is rooted in the current realities of trial execution and informed by direct engagement with emerging best practices.
Stay tuned for our next SNMMI mini-series post, where we’ll explore how radiopharmaceutical maturity is influencing investor expectations and system-level readiness.
Read the full SNMMI mini-series here:
1. From the SNMMI Frontlines: 5 Takeaways Reshaping Radiopharmaceutical Strategy
4. Neurology’s Radiotheragnostic Turn: SNMMI Signals an Imaging-Led Therapeutic Revolution
References:
[1] UroToday, 2025. SNMMI 2025: Clinical Trial Protocol Update – FLEX-MRT. A randomized trial evaluating flexible vs. fixed dosing of ¹⁷⁷Lu-PSMA-617 in mCRPC. UroToday.
[2] UroToday, 2025. SNMMI 2025: First-in-Human Results of Terbium-161 (¹⁶¹Tb PSMA I&T) Radioligand Treatment – VIOLET Study. Safety and preliminary efficacy of ¹⁶¹Tb-PSMA-I&T in advanced prostate cancer. UroToday.
[3] UroToday, 2025. SNMMI 2025: Prognostic Evaluation of an Artificial Intelligence–based Risk Score. AI-derived PSMA PET/CT scoring model for survival prediction. UroToday.
[4] UroToday, 2025. SNMMI 2025: WB SPECT/CT Studies Post Lu 177 Therapy. Quantitative imaging for treatment response and progression prediction. UroToday.
[5] UroToday, 2025. SNMMI 2025: Sequencing and Combining Beta and Alpha Targeted Radionuclide Therapies in Prostate Cancer. Multimodal trial strategies to overcome resistance and optimize duration of benefit. UroToday.
[6] ClinicalTrials.gov, 2025. Phase 2 Randomized Trial of Flexible Dosing Schedule of ¹⁷⁷Lu-PSMA-617 for the Treatment of Metastatic Castration-Resistant Prostate Cancer (FLEX-MRT). Adaptive dosing trial comparing fixed vs. extended Pluvicto® schedules in mCRPC. ClinicalTrials.gov
[7] Buteau JP, Kostos L, Alipour R, et al., 2024. VIOLET Trial Protocol: Radioligand Therapy with ¹⁶¹Tb-PSMA-I&T in mCRPC. Safety and preliminary efficacy of terbium-based PSMA radioligand in prostate cancer. Journal of Nuclear Medicine. JNM
[8] ClinicalTrials.gov, 2025. Evaluation of Radioligand Treatment in mCRPC with ¹⁶¹Tb-PSMA-I&T. Phase I/II trial evaluating safety and dosing of terbium-labeled PSMA therapy in metastatic prostate cancer. ClinicalTrials.gov.
[9] Yadav S, Lowery B, Moradi Tuchayi A, et al., 2024. Impact of Posttreatment SPECT/CT on Patient Management During ¹⁷⁷Lu-PSMA-617 Radiopharmaceutical Therapy. SPECT/CT findings altered management in 49% of patients, most often at cycles 2 and 4. Journal of Nuclear Medicine. JNM
